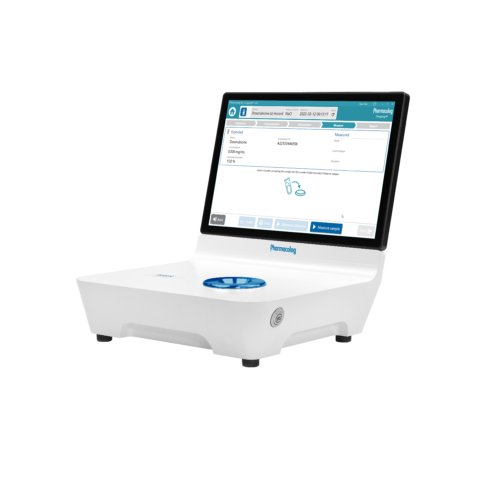
Kvalitetskontroll av beredda läkemedel
Vi har ett ansvar att säkerställa patientsäkerhet och läkemedelseffekt inom vårt hälso- och sjukvårdssystem. DrugLog™ är en kostnadseffektiv lösning för att snabbt och effektivt verifiera identitet och koncentration hos beredda injicerbara läkemedel. Den snabba, tillförlitliga och användarvänliga DrugLog™-enheten verifierar rätt läkemedel och rätt koncentration på några sekunder och blir en ovärderlig del av er kvalitetskontrollprocess.

Minska läkemedelsfel
DrugLog™ är en robust fristående lösning för att minska beredningsfel. Den verifierar identitet och koncentration hos beredda injicerbara läkemedel innan de administreras till patient. Oavsett om DrugLog™ används på apoteket eller på vårdavdelningen kan ni känna er trygga med att patienten får det som avsågs.
Extra försiktighet, ökad säkerhet
DrugLog™ är ett utmärkt komplement till de redan höga säkerhetskraven vid läkemedelsberedning. De flesta cytostatika som används vid cancerbehandling bereds individuellt på sjukhusapotek. Även i händerna på den mest erfarna professionella finns alltid en risk för fel. Läkemedelsberedning kräver att extra försiktighetsåtgärder vidtas genom hela processen. DrugLog™ eliminerar osäkerheten i kvalitetskontrollen och hjälper er att uppfylla regelkraven.

Nyckelfunktioner i DrugLog™

Tillförlitlig läkemedelsverifiering på sekunder
DrugLog™ är en unik kombination av banbrytande programvara och beprövad, tillförlitlig hårdvara för absorptionsspektroskopi. Inom några sekunder kan användaren avgöra om en spädd eller beredd produkt är rätt läkemedel och har rätt koncentration.
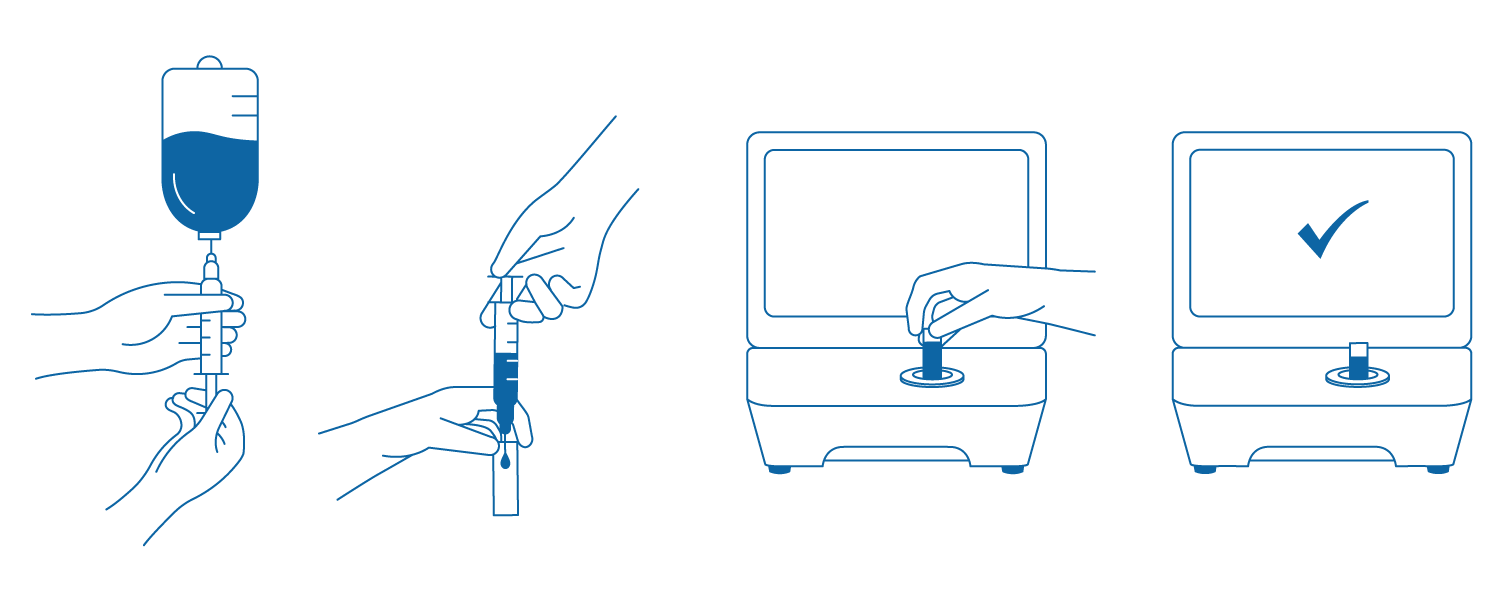
Så fungerar det
1. Dra upp ett litet prov (0,3–0,5 ml) från den beredda lösningen.
2. Injicera provlösningen i kuvetten.
3. Sätt in kuvetten i DrugLog™-enheten.
4. Starta testet och se resultatet inom 2–3 sekunder.
DrugLog™ är flexibel för olika beredningsflöden
Oavsett om ni gör stickprovskontroller av batchberedningar eller genomför oberoende verifiering av en beredningsrobot efter underhåll eller programvaruuppgradering, ger DrugLog™ trygghet i att roboten fungerar som den ska. DrugLog™ skapar konsekvens i manuella beredningsprocesser och är ett utmärkt verktyg vid personalutvärderingar. Den är även idealisk för verifiering av externt beredda läkemedel vid mottagande.
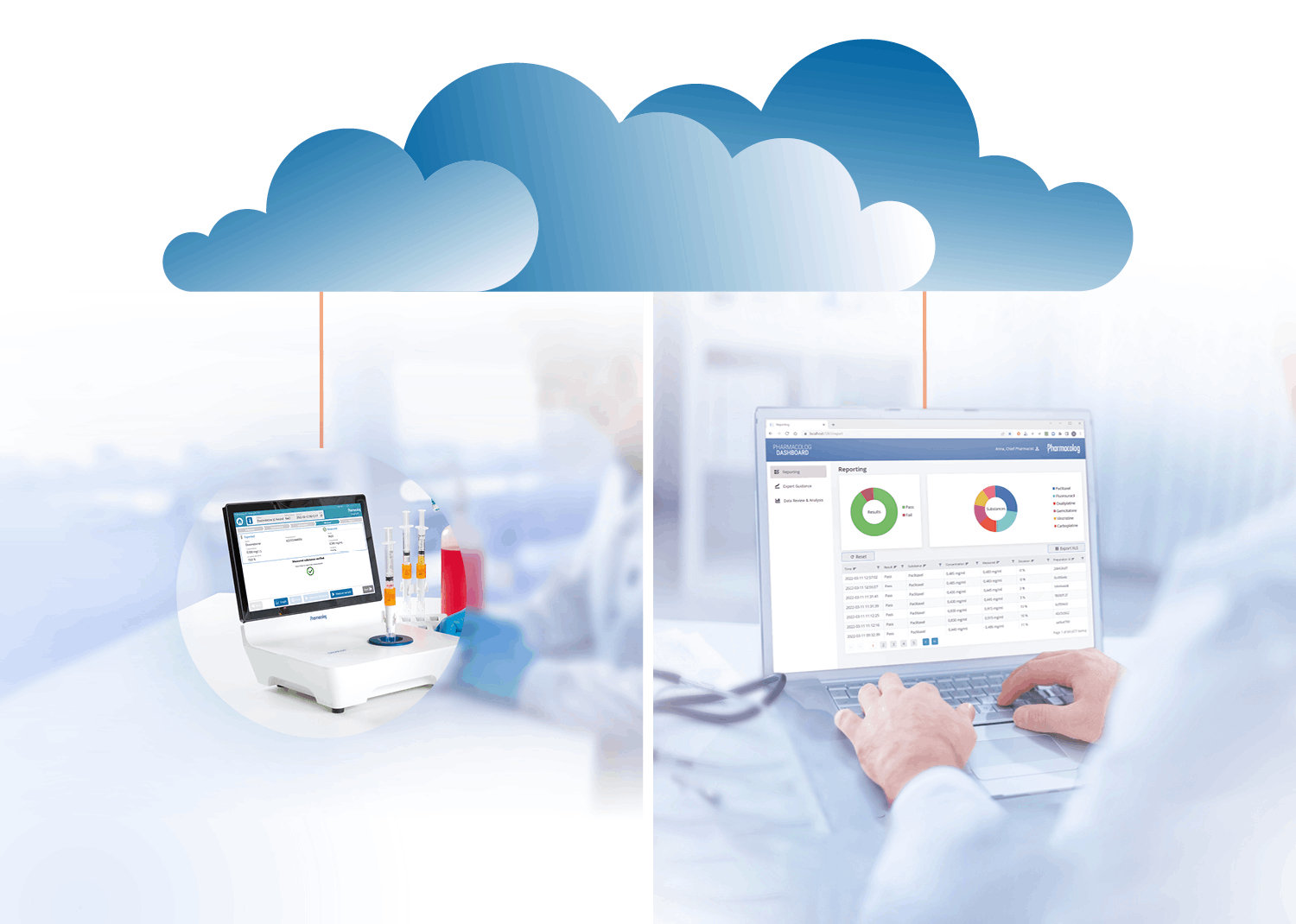
Få tillgång till data för granskning och analys
Pharmacolog Dashboard™ ger fjärråtkomst till en heltäckande översikt över alla läkemedelsverifieringar. Bland många fördelar kan en senior farmaceut exempelvis på distans stödja kollegor i komplexa beredningsprocesser i renrummet utan att vara fysiskt närvarande på apoteket.

Varför DrugLog™?
Dokumentation

DrugLog™
Broschyr
Ladda ner PDF

Pharmacolog Dashboard™
Broschyr
Ladda ner PDF

Pharmacolog
Service & Support
Broschyr
Ladda ner PDF

Kundberättelse –
Cliniques Universitaires Saint-Luc, Bryssel, Belgien
Ladda ner PDF
